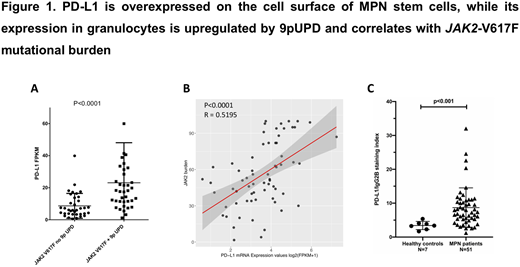
Myeloproliferative neoplasms (MPN) are characterized by clonal hematopoiesis, hyperproliferation of myeloid cells, hyperinflammation and immune deregulation. The three classical BCR-ABL1-negative MPN are essential thrombocythemia (ET), polycythemia vera (PV) and primary myelofibrosis (PMF). The disease is driven by JAK2, CALR or MPL somatic mutations in most patients. Drug resistance is a major problem in MPN. Recent data suggest that MPN cells display certain immune checkpoint molecules that may contribute to resistance, including PD-L1. Antibodies targeting the PD1/PD-L1 axis are highly promising anti-cancer drugs. Their potential use in MPN is being explored but it is unclear which MPN subtypes are most suitable for testing in clinical trials. The aim of our project was to assess PD-L1 expression in disease-initiating neoplastic stem cells (SC) and differentiated cells of MPN patients and to develop therapeutic approaches capable of blocking PD-L1 expression in MPN SC. In a first step, PD-L1 expression was assessed by RNA-sequencing of granulocytes of 106 MPN patients and 15 healthy donors (HD). The cohort included 56 PMF, 33 ET and 17 PV patients. For 102 patients data from Human Genome-wide Affymetrix 6.0 SNP arrays were available. We observed a ~5-fold higher expression of PD-L1 mRNA in patients with PV compared to other MPN (P<.01) or HD (P<.01). JAK2-V617F positive ET patients had higher expression of PD-L1 compared to CALR-mutated ET (p<.005) and the same was observed in PMF (p<.01). Other mutations (TET2, DNMT3A) detected by NGS did not affect PD-L1 expression. Since PD-L1 and JAK2 are located on chromosome 9p24, we looked into our previously published dataset of 400 MPN patients analyzed by SNP arrays and found that in all 195 patients with 9p uniparental disomy (UPD) the aberrations covered both genes. As PD-L1 is more centromeric it could represent the second target of 9pUPD which can precede the acquisition of JAK2-V617F in MPN. Granulocytes in JAK2-V617F positive patients with 9pUPD expressed significantly higher levels of PD-L1 compared to patients without 9pUPD (P<.0001; Figure 1A). Moreover, the JAK2-V617F mutational burden significantly correlated with PD-L1 expression (R=.52, P<.0001; Figure 1B). This correlation was lost when cases with 9pUPD were excluded from the analysis (R=.03, P=.9), indicating that the UPD is relevant for PD-L1 upregulation. To investigate PD-L1 surface expression on MPN SC we analyzed CD34+CD45dimCD38- cells isolated from fresh bone marrow (BM) samples of another 51 MPN patients and 7 HD by flow cytometry (FC). MPN patients showed a significantly higher surface expression of PD-L1 on CD34+CD45dimCD38- cells compared to HD (p<.001; Figure 1C). PD-L1 levels on the SC surface were elevated in both JAK2- and CALR-mutated MPN patients compared to HD (p<.001 and P<.005, respectively). PD-L2 was neither expressed in MPN granulocytes nor on MPN SC. CD4+ and CD8+ T-cells from BM samples of 17 MPN patients expressed the PD-L1 receptor PD-1 as assessed by FC. We cultured ex vivo primary MPN cells from 7 JAK2-V617F positive patients and showed that PD-L1 expression on MPN SC spontaneously decreases in culture, that interferon-gamma (IFN-γ) can promote expression of PD-L1 on these cells, and that ruxolitinib and the BRD4-degrader dBET6 block IFN-γ-induced PD-L1 expression in CD34+CD45dimCD38- MPN SC (P<.05). Together, we show that PD-L1 is overexpressed on the surface of disease-initiating MPN SC, that PD-L1 mRNA is overexpressed in granulocytes of MPN patients and that PD-L1 overexpression in granulocytes correlates with the JAK2-V617F mutational burden. In patients with JAK2-V617F positive MPN, 9pUPD leads to further PD-L1 upregulation either through increasing the mutant JAK2 gene dosage, loss of wt-JAK2,or amplification of PD-L1 allele with higher expression. Our data suggest the possibility that 9pUPD and the subsequent elevation of PD-L1 expression may provide an immune escape mechanism and may contribute to positive selection of JAK2-V617F homozygous SC. Ruxolitinib and dBET6 downregulate PD-L1 expression on MPN SC suggesting a role for the JAK2 and BRD4-MYC pathway. As recent studies revealed an immunogenic potential of JAK2 and CALR mutants, overcoming the disease-mediated immune escape may be of particular importance. Further preclinical and clinical studies are now required to examine the value of PD1/PD-L1 inhibitors in patients with MPN.
Gisslinger:Celgene: Honoraria; MyeloPro Diagnostics and Research: Honoraria; AOP Orphan Pharmaceuticals AG: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; PharmaEssentia: Honoraria; Janssen-Cilag: Honoraria; Roche: Honoraria. Kralovics:AOP Orphan Pharmaceuticals AG: Honoraria; PharmaEssentia: Honoraria; Qiagen: Honoraria; Novartis: Honoraria; MyeloPro Diagnostics and Research: Current equity holder in private company. Valent:Allcyte GmbH: Research Funding; Pfizer: Honoraria; Cellgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal